CONDUCTOMETRY
Definition:
Conductometry is an electroanalytical technique that measures the electrical conductivity of a solution to monitor changes during a chemical reaction especially during titrations.
Why We Use Conductometry for Endpoint Detection in Titration?
Conductometric titration is used for endpoint detection due to its objectivity, precision, and adaptability:
- No need for visual indicators – ideal for colored or opaque solutions.
- High sensitivity to ionic concentration changes – even for dilute or weak systems.
- Applicable to non-acid-base systems like precipitation, redox, and complexometric titrations.
- Suitable for automation – perfect for modern analytical labs and process control.
- Accuracy in difficult conditions – such as high temperatures, colored mixtures, or systems without clear pH shifts.
Types Of Titrations Where Conductometry Is Used:
| TYPE | EXAMPLE | CONDUCTIVITY CHANGE PATTERN |
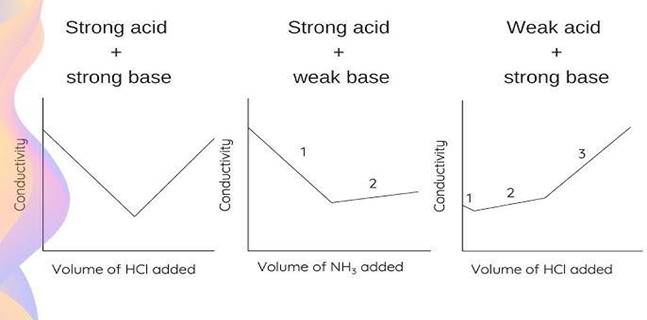
| Stong Acid-Strong Base | HCL Vs NaOH | Decrease→ Minimum→ In crease |
| Weak Acid-Strong Base | CH3COOH Vs NaOH | Gradual decrease, then sharp increase |
| Strong Acid-Weak Base | HCL Vs NH4OH | Steep Decrease→ Slight Increase |
| Precipitation Titration | AgNO3 Vs NaCL | Sudden Drop (Ion Removed)→ Rise (Excess Titrant Ions) |
| Redox Titration | KMnO4 Vs H2C2O4 (Oxalic Acid) | Conductivity varies with oxidation states and products |
| Complexometric Titration (used when indicator is unsuitable) | EDTA Vs Ca2+ or Mg2+ | Gradual conductivity drops |
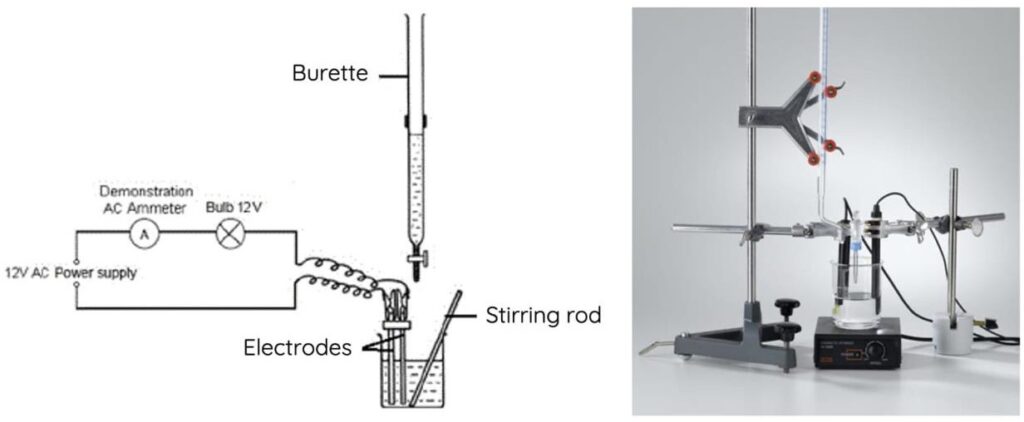
Mechanism: How Endpoint Detects in Titration Using Conductometry
The principle behind conductometric titration lies in the variation in ionic concentration that influences the conductivity of solution.
- Setup and baseline reading: Measure initial conductivity based on ions present.
- Upon addition of titrant: Analyte-titrant reaction changes ionic species.
- Ion mobility effect: Highly mobile ions are replaced by less mobile ones, causing conductivity to drop.
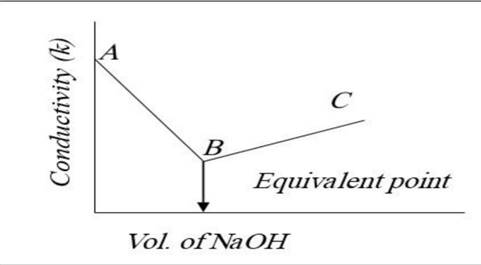
- At equivalence point: At stoichiometric completion, conductivity shows a distinct minimum, change in slope, or inflection means equivalence reached.
- Post equivalence point: Excess titrant cause rise in conductivity again.
For instance, in titration of HCl against NaOH:
- At the beginning, H⁺ ions are responsible for high conductivity.
- When NaOH is added, the H⁺ ions are consumed to form water (non-conductive), and Na⁺ ions take their place, leading to a decrease in conductivity.
- After the equivalence point, OH⁻ ions from excess NaOH raise conductivity again.
HCL + NaOH → H2O + NaCL
Applications: Conductometry is a powerful analytical technique that measures the electrical conductivity of ionic solutions. It finds wide application across laboratory and industrial settings due to its versatility and reliability, especially in situations where conventional indicators fail. Below are its major applications:
1. Titration in Colored, Turbid, or Indicator-Incompatible Solutions:
In cases where visual indicators are ineffective—such as colored, turbid, or transparent solutions with minimal pH changes—conductometric titration offers a reliable alternative by detecting changes in conductivity rather than relying on color change.
2. Weak Acid–Base and Non-Standard Reactions:
Conductometry is especially useful in weak acid–base titrations where minimal pH variation makes indicators unreliable. It is also applicable in reactions lacking suitable indicators or involving non-aqueous media.
3. Determination of Salt Concentrations and Solubility of Sparingly Soluble Salts:
It accurately monitors precipitation titrations (e.g., AgNO₃ vs NaCl) and determines the solubility of salts like barium or lead sulfate by measuring ion release and changes in conductivity.
4. Water Quality Assessment: Purity, Salinity, and Alkalinity:
Conductometry is widely used to:
- Assess the purity of distilled or demineralized water (low conductivity indicates high purity).
- Evaluate salinity in seawater and alkalinity in freshwater by detecting ionic concentrations.
5. Chemical Equilibria and Organic Compound Analysis:
It aids in:
- Determining the ionic product of water (Kw).
- Studying ionic equilibria during chemical reactions.
- Analyzing organic acids for basicity and quantifying special compounds such as deuterium in heavy water.
6. Industrial and Automated Quality Control:
Conductometric titrations are easily automated, making them ideal for routine quality checks in the pharmaceutical, food, and water treatment industries where precision and speed are essential
Applications of Conductometry in Chemistry and Industry
Highlight practical uses:
- Colored/turbid solutions
- Weak acid–base titrations
- Salt solubility
- Water quality
- Organic compound analysis
- Automated quality control in labs
6. Key Advantages of Conductometric Titrations
Summarize the benefits:
- No indicator needed
- High precision
- Ideal for automation
- Works in difficult conditions
7. Conclusion: Why Conductometry Matters in Modern Analytical Chemistry
Conductometry offers a powerful, precise, and non-visual method for tracking chemical reactions. Its adaptability to weak systems, turbid mixtures, and automation makes it indispensable in analytical labs today.
